Written by Alexandra Putka
Edited by Dr. Larissa Nitschke
Profound gene changes in non-neuronal cells are revealed by a recent study in SCA1.
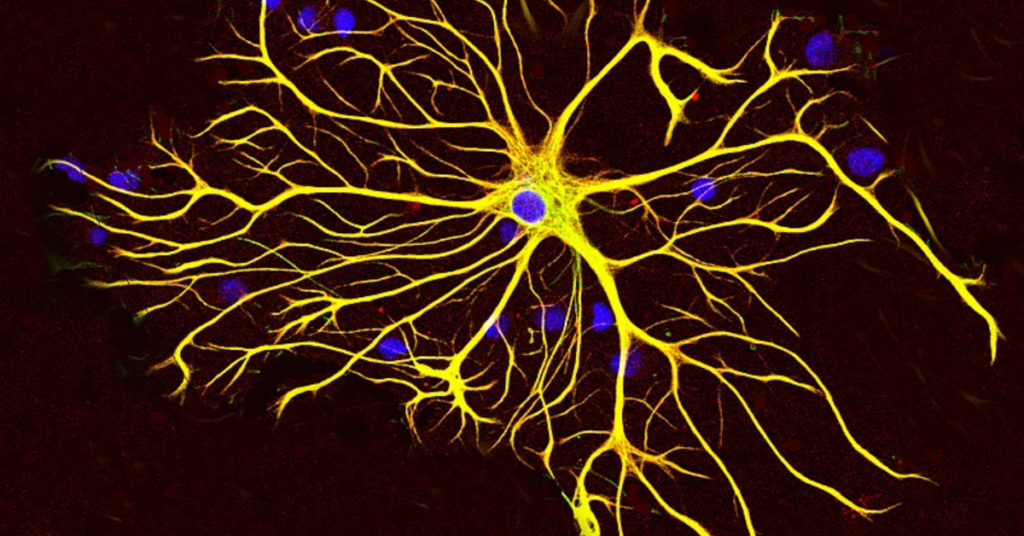
The two major cell types of the brain are neurons and glia, yet you have probably heard little about glia, which outnumber neurons. The word glia comes from the Greek word for glue, as these cells were traditionally thought to hold the brain together and play a secondary role to neurons. However, glia are quickly gaining recognition in neurodegenerative disease research, and spinocerebellar ataxia type 1 (SCA1) is no exception. In a recent study by Borgenheimer and colleagues, researchers show that glial cell genes are changed in the cerebellum of an SCA1 mouse model. This opens up new avenues of research to better understand the role of glia in SCA1 and how they could be targeted for therapeutic benefit.
SCA1 is a dominantly inherited progressive cerebellar ataxia caused by an expanded CAG repeat in the ATXN1 gene. The present study sought to determine which genes are changed in neuronal and glial cells in an early-stage SCA1 mouse model. To do this, the scientists used a mouse model expressing the human mutant ATXN1 gene. Here, mutant means that the gene contains the expanded CAG repeat. This mouse model expresses mutant ATXN1 in only one affected cell type in SCA1: the Purkinje cells of the cerebellum. Purkinje cells are the output neurons of the cerebellum, as they transmit information to the rest of the body to enable smooth movement. While this mouse model does not perfectly represent the human disease in which mutant ATXN1 is present in all cell types and brain regions, it helps the authors answer their specific question of how glia are affected by diseased Purkinje cells. If mutant ATXN1 were to be present in all cell types, the authors would not have been able to distinguish if gene changes in glial cells were due to 1) mutant ATXN1 wreaking havoc on these cells or 2) Purkinje cell abnormalities affecting the glial cells. By using the mouse model with ATXN1 only in Purkinje cells, the authors are able to selectively understand how Purkinje cell dysfunction affects glia in SCA1.
The researchers used a technique called single-nuclei RNA-sequencing to uncover gene changes in the cerebellum of their mouse model. They studied mice at 12 weeks of age, which represents an early disease time point at which the disease is still reversible and could be targeted therapeutically. In this study, single-nuclei RNA-sequencing involved taking the cerebellum of control and SCA1 mice and separating out individual cell types. The authors then analyzed the genes expressed in each of these cell types, specifically using the nucleus of the cell because this is where the genetic information is held. Think of the cerebellum as a smoothie with many different cell types (fruits). In order to appreciate what each fruit adds to the smoothie, you need to study each individually. That is what the authors accomplish with their single-nucleus method: studying the genetic information in each individual cell to better understand how each cell type functions in disease. This method is becoming increasingly popular in neurodegenerative disease research to give researchers insight into what cell types are affected in disease and what genes could be targeted with therapeutic approaches.
The authors focused on gene changes in neurons (Purkinje cells) and glia of the cerebellum. More specifically, they investigated two types of glial cells: 1) astrocytes (including cells called velate astrocytes and Bergmann glia, found in different layers of the cerebellum) and 2) oligodendrocytes. Briefly, astrocytes provide structural and functional support to neurons while oligodendrocytes wrap around the neuron to speed up electrical transmission of information. The researchers found genes that were changed between SCA1 and control mice in each of these cell types. Starting with Purkinje cells, which are well-described in SCA1, the authors uncovered novel increases in a gene that is protective in Alzheimer’s disease and genes related to neuronal information transmission. This suggests that Purkinje cells increase the production of genes that defend against disease as a reaction to mutant ATNX1-induced cellular toxicity. Protective genes are also increased in velate astrocytes, indicating that these cells react to Purkinje cell dysfunction. In the other astrocyte subtype, Bergmann glia, the authors tease apart Purkinje cell-Bergmann glia connections. They find alterations in the pathway these cells use to communicate, called Sonic hedgehog (yes, named after the video game character). Finally, oligodendrocytes had gene changes related to altered cognition and locomotion. This is particularly interesting because it suggests that oligodendrocyte dysfunction may be connected to SCA1 symptoms.
The study further investigates gene changes that were shared by these cell types. Such genes represent widespread alterations in disease that could be investigated for therapeutic benefit. One example of a shared pathway is calcium signaling, which is vital for a number of cellular processes including neuron signal transmission. Another shared alteration occurred in the retinol (vitamin A1) pathway, specifically in Transthyretin which transports thyroid hormones and retinol. Therefore, restoring calcium signaling or supplying Transthyretin should be further investigated in mouse models of SCA1 to determine if these gene changes could produce symptomatic improvement.
In conclusion, this study pushes the field forward by providing a wealth of information on gene alterations in SCA1 Purkinje cells and glia. This not only helps identify dysfunctional pathways in disease, but also highlights the role of protective genes that are increased to correct pathway impairments. Since the authors used an early-stage mouse model, the observed changes occur early enough to be potential targets for treatment. The scientists also highlight that expression of mutant ATXN1 in Purkinje cells does not only affect these cells, but also other surrounding cells, including astrocytes and oligodendrocytes. This means that all of these cells should be further researched to determine how they contribute to disease and whether they could be targets of treatment. Therefore, the study expands upon what we know about Purkinje cell abnormalities in disease and increases knowledge on the role of glial cells in SCA1.
Key Words
Single-nuclei RNA-sequencing: A method allowing researchers to isolate the nucleus from each cell within a tissue sample and then study the RNA within that individual cell. During analysis, genetic information is grouped by cell type to understand cell-type-specific gene changes in disease. See entry for RNA-sequencing for more details on this method.
Glia: the other cell type of the brain besides neurons. Divided into subcategories astrocytes, microglia, and oligodendrocytes. These cells provide structural and functional support to neurons and have functions of their own not related to neuronal support. They are known to play important roles in many neurodegenerative diseases.
Oligodendrocyte: A cell type which wraps a fatty coating called myelin around neurons to provide electrical insulation.
Bergmann glia: A type of astrocyte found within the Purkinje cell layer of the cerebellum. They are structurally and functionally connected to Purkinje cells.
Velate astrocyte: A type of astrocyte found within the granular layer of the cerebellum. Little is known about how they respond to cerebellar disease.
Conflict of Interest Statement
The author and editor declare no conflict of interest.
Citation of Article Reviewed
Borgenheimer E, Hamel K, Sheeler C, Moncada FL, Sbrocco K, Zhang Y, Cvetanovic M. Single nuclei RNA sequencing investigation of the Purkinje cell and glial changes in the cerebellum of transgenic Spinocerebellar ataxia type 1 mice. Frontiers in Cellular Neuroscience, 2022. 998408 (16). (https://pubmed.ncbi.nlm.nih.gov/36457352/)
Read Other SCAsource Summary Articles

¿Ser o no ser SCA27B? Esa es la cuestión: Misteriosos casos de ataxia resueltos gracias a los avances en tecnología genética
Escrito por Dr. Hayley McLoughlin y Dr. Sharan SrinivasanEditado por Dr. Celeste SuartTraducido por Ismael Araujo-Aliaga Una nueva tecnología de secuenciación genética reveló numerosos casos de ataxia familiar asociados a Read More…


Interrupciones en las repeticiones están asociadas con ataques epilépticos en SCA10
Escrito por Dr Hannah ShorrockEditado por Larissa NitschkeTraducido por Ismael Araujo-Aliaga Interrupciones en las repeticiones en SCA10 influyen en la estabilidad del tramo repetido y están asociadas con ataques epilépticos Read More…


Genetic variants in the NPTX1 gene cause cerebellar ataxia
Written by Dr. Hannah K Shorrock Edited by Dr. Celeste Suart Three genetic variants in the NPTX1 gene have been linked to cerebellar ataxia, providing a genetic diagnosis for seven Read More…









