NAF to Award $735,000 in Ataxia research grants in 2023
There was a robust pool of applicants for NAF research grants in 2023. It demonstrates the energy to accelerate treatment development in this space. We were thrilled to see so many studies with high scientific merit! These grants allow Ataxia investigators to request funding for their studies to lead us closer to possible treatments.

This year’s funded projects will support research on 11+ genetic forms of Ataxia, from the most common to some of the rarest, as well as address shared disease pathways that could provide insights into ALL of the Ataxias.
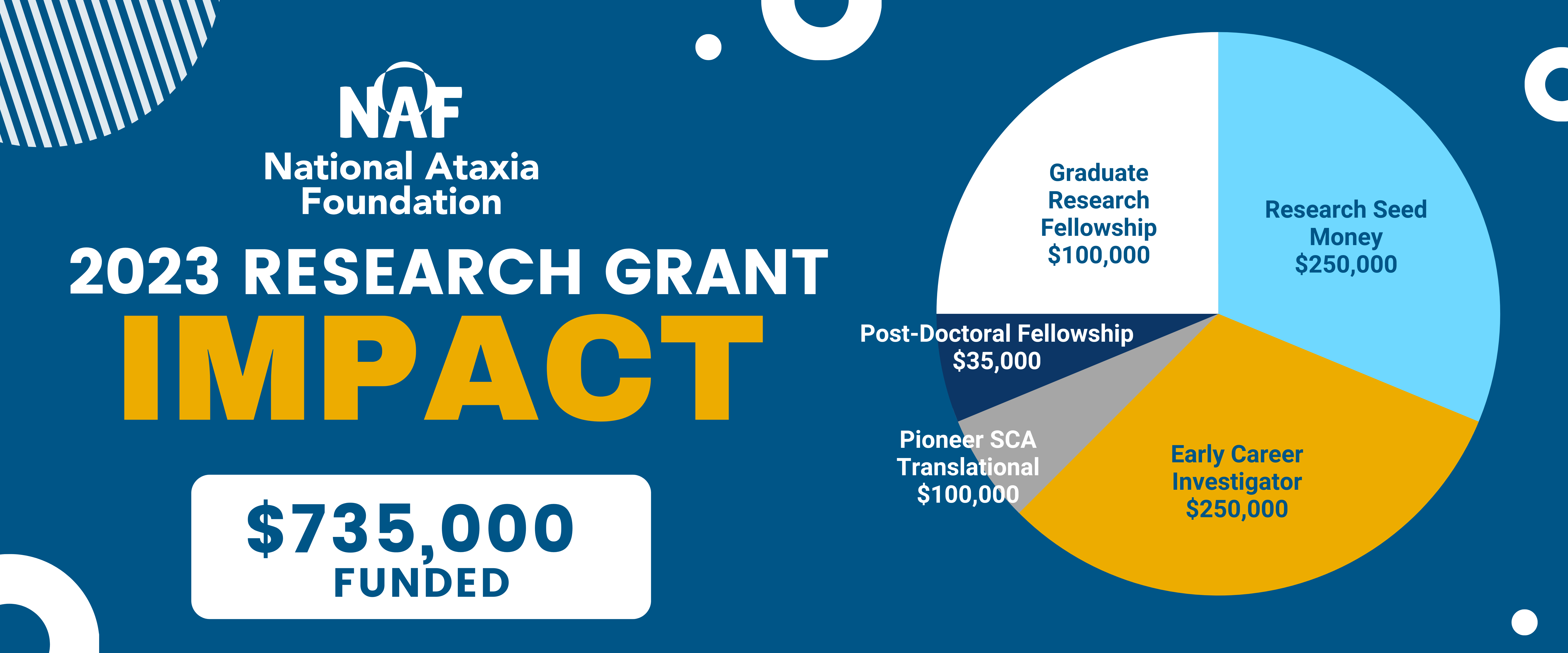
For 2023, NAF is funding:
- 5 Research Seed Money Grants – totaling $250,000
- 5 Early Career Investigator Awards – totaling $250,000
- 4 Graduate Research Fellowships – totaling $100,000
- 1 Pioneer SCA Translational Award – totaling $100,000
- 1 Post-Doctoral Fellowship – totaling $35,000
A full list of the research studies and their lay summaries are available below.
Lay Summaries
Research Seed Money Grants

Dr. Brendan Battersby
University of Helsinki, Finland
Investigating sources of proteotoxicity substrates with AFG3L2/SPG7 ataxia disorders
Ataxia Type: Mitochondrial ataxia with autosomal recessive and dominant origins
Our research investigates inherited ataxia disorders that arise from disruptions to cellular energy production. The generation of misfolded proteins needed for energy production is an initial event in the disease. The goal of the Seed Funding from NAF is to identify the sources of misfolded proteins and to limit their production as a therapeutic target.

Dr. Hayley McLoughlin
University of Michigan Medical Center (Michigan Medicine)

Dr. Janghoo Lim
Yale University
Mapping longitudinal single-cell transcriptional dynamic vulnerabilities in SCA3 cerebella
Ataxia Type: SCA3
This proposal seeks seed funding support to map longitudinal single-cell transcriptional dynamics in vulnerable Spinocerebellar ataxia type 3 (SCA3) mouse cerebella. We will dissect how individual cell types within a heterogenous tissue contribute to pathogenesis. Furthermore, we will relate the transcriptional changes seen in SCA3 mice to single-nuclear RNA sequencing we have generated from human SCA3 cerebella. Together, we hope to uncover new roles for diverse cerebellar cell types in SCA3 and identify the molecular events underlying disease that may elucidate protective pathways and biomarkers for SCA3.

Dr. Thorsten Schmidt
University of Tübingen
Dissecting the role of O-GlcNAcylation in polyglutamine-related spinocerebellar ataxias
Ataxia Type: Spinocerebellar ataxias caused by Polyglutamine expansions
We recently identified that the ataxin-3 protein, which is mutated in Spinocerebellar ataxia Type 3, is involved a cellular protein modification process in which sugar molecules are added to proteins.
Influencing this process alleviated important disease processes.
We will now dissect the role of this modification process in other ataxias in order to identify whether our promising results can be applied to a larger group of ataxias.

Dr. Alanna Watt
The Royal Institution for the Advancement of Learning/McGill University
Biological basis for circadian disruption in SCA6
Ataxia Type: SCA6
Most of us know that sleep is one of the best medicines. However, sleep disturbances are common in ataxia, making the promise of a good night’s sleep elusive for many ataxic patients.
SCA6 is a form of ataxia where sleep disruption has been reported. We study a mouse model of SCA6 and report that these mice show disruption in their sleep/wake activity patterns, as well as a downregulation in a brain receptor that is known to modulate the “molecular clock” that organizes the sleep/wake cycle in organisms. This proposal aims to uncover the biological basis for sleep deficits in SCA6.

Dr. Ricardo Mouro Pinto
Massachusetts General Hospital
Genetic modifiers of somatic CAG expansions in spinocerebellar ataxia type 1
Ataxia Type: SCA1
Spinocerebellar ataxia 1 (SCA1) is caused by an expanded CAG repeat in the ATXN1 gene, with longer repeats being associated with earlier disease onset and worst symptoms. A hallmark of most repeat expansion disorders, including SCA1, is that the repeats are highly unstable, not only when transmitted from parent to child, but also within a patient’s brain, where the repeat keeps expanding over time – aka somatic expansion. Somatic expansions significantly affect disease onset in another disorder also caused by a CAG repeat expansion – Huntington’s disease (HD). The mechanism underlying this remains poorly understood in SCA1. In this study we will test if know genetic modifiers of repeat instability in HD are also involved in regulating somatic CAG expansions in SCA1. Knowing which genes also play a role in SCA1 somatic expansions will lead to identification of potential therapeutic targets.
Pioneer SCA Translational Research Award

Dr. Roderick Maas
Stichting Radboud Universitair Medisch Centrum (Radboudumc)
Looking beyond the central nervous system in SCA3: nerve and muscle ultrasound as potential imaging markers to quantify and monitor peripheral nervous system degeneration
Ataxia Type: SCA3
Besides ataxia, patients with SCA3 frequently report bothersome muscle cramps, pain, tingling/numbness, and loss of muscle mass due to concomitant involvement of various peripheral nervous system structures. In this study, we will use ultrasound to visualize (changes in) nerves and muscles of individuals with SCA3 across the disease spectrum, including pre-ataxic mutation carriers. Specifically, we will investigate if nerve sizes, muscle thickness, and muscle structures differ between SCA3 patients and healthy controls, but also if ultrasound abnormalities already can be detected before the onset of ataxia and if these progress over time at a 1-year follow-up visit.
Early Career Investigator Awards

Dr. Jarmon Lees
St Vincent’s Institute of Medical Research
MEG3 and TRIM4 as novel therapeutic targets for vascular dysfunction in Friedreich ataxia
Ataxia Type: Friedreich’s Ataxia
For sufferers of the rare, degenerative disease Friedreich ataxia, heart disease is a life-threatening complication. This project uses stem cells from people with Friedreich ataxia to create a “heart-in-a-dish” which has allowed us to identify two genes which may contribute to heart disease. Our goal is to test if forcibly reducing the expression of these two genes can protect the heart tissue and restore healthy beating.

Dr. Sasha Zhang
The Regents of the University of Michigan
Metformin as potential therapeutics in FXTAS
Ataxia Type: Fragile X-associated tremor/ataxia syndrome (FXTAS)
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder that affects upwards of one in 500 people over the age of 50 worldwide. The disease is caused by a CGG repeat expansion that gets translated into toxic proteins through an unusual process known as RAN translation. Metformin is an FDA approved and widely prescribed drug that suppresses RAN translation in a model of ALS. With support from NAF, we will assess whether Metformin might also be beneficial in preclinical models of FXTAS as a precursor for clinical trials in patients.

Dr. Dongwon Lee
Baylor College of Medicine
Identifying molecular mechanisms of cerebellar hypoplasia and ataxia in CASK disorder
Ataxia Type: Cerebellar Ataxias
CASK disorder caused by mutations of the CASK gene has cerebellar hypoplasia and ataxia, however its pathogenesis is unknown. This proposal aims to elucidate molecular mechanisms underlying cerebellar granule cell death in CASK disorder mouse model. We expect to use this knowledge to develop new therapeutic strategies that will prevent the pathological cell death in cerebellar hypoplasia and ataxia in CASK disorder.

Dr. Simone Mader
LMU University Hospital Munich
Novel brain reactive autoantibodies directed to synaptic proteins in cerebellar ataxia
Ataxia Type: Immune-mediated cerebellar ataxia
In the last years, several autoantibodies have been discovered, which can serve as important biomarkers and may guide therapeutic interventions, but for many patients with cerebellar ataxia, the precise pathomechanisms and the involvement of autoantibodies is unclear. With the support from NAF, we aim to characterize novel synaptic antibodies and understand their frequencies in cerebellar ataxia.
As emerging cases of antibody-mediated cognitive and behavioral impairments are identified in cerebellar ataxia and we continue to gain deeper insights on environmental factors that may trigger brain-reactive antibody response, the approach of preventing pathogenic antibodies from binding to their tissue targets may be used therapeutically in at-risk individuals.
Results Update (Feb 2025):
Immune-mediated cerebellar ataxia is associated with various autoimmune neurological disorders. While some cases have a known cause, many are still considered idiopathic. Recent studies have shown that autoantibodies, which target specific brain components, can be found in people with autoimmune neurological diseases. These autoantibodies may provide useful markers for better diagnosis and treatment. In our research, we found that 10% of patients with idiopathic cerebellar ataxia had a distinct pattern of autoantibody reactivity against cerebellar tissue. By examining cells from the patient’s cerebrospinal fluid, we were able to create monoclonal antibodies that mirrored the serum autoantibody reactivity pattern. Identifying the exact targets of these antibodies in the future could lead to improved diagnostic methods and a better understanding of the condition, potentially offering new treatment options for patients with currently unexplained ataxia.

Dr. Hirofumi Fujita
The University of Texas Southwestern Medical Center (UT Southwestern)
Defining cell type-dependent vulnerability of the dentate and Purkinje neurons in SCA3
Ataxia Type: SCA3
While the pathology of SCA3 has primarily been investigated by focusing on the cerebellar cortex, SCA3 is also known for the prominent loss of the neurons in the cerebellar nucleus, a region responsible for mediating the information from the cerebellar cortex to the rest of the brain. In this project, by using SCA3 mutant mice, we focus on the dentate nucleus, a prominent part of the cerebellar nucleus that is known to degenerate in SCA3, to examine which specific dentate neurons and their connections from the cerebellar cortex are susceptible to this disease. With this project, we hope to start to understand the circuit pathology of the SCA3.
Post-Doctoral Fellowship

Dr. Stéphanie Oliveira
University of Minho
The multimodal antidepressant vortioxetine as a therapeutic strategy to mitigate SCA3
Ataxia Type: SCA3
My team previously showed that prolonged treatment with an antidepressant called citalopram, which increases serotonin levels in the brain, improved motor symptoms and reduced the amount of toxic ataxin-3 protein of SCA3 animal models. This work showed that targeting the serotonin systems could be a successful treatment option for SCA3. Given these promising results testing citalopram in SCA3, I will now investigate the effect of further increasing serotonin action in the brain by using a different antidepressant, called vortioxetine. Vortioxetine simultaneously targets a number of pathways within the brain, which work together to further increase the amount and activity of serotonin. We think that by using vortioxetine, an even greater reduction in motor symptoms in SCA3 animal models can be achieved.
NAF Graduate Research Fellowship

Ran Ming
Duke University
Structural-Function Elucidation of Spinocerebellar Ataxia Type 48 (SCA48)
Ataxia Type: SCA48
Spinocerebellar Ataxia type 48 (SCA48) is a recently discovered form of SCA that is caused by mutations to the protein C-terminus of Hsc70 Interacting Protein (CHIP). The focus of this fellowship is to understand how these mutations cause defects in CHIP function that result in SCA48. The studies proposed in this fellowship will increase our understanding of SCA48 and may lead to the identification of novel therapeutic targets.

Jason (Zexin) Li
The Regents of the University of Michigan
Identification and characterization of a novel ataxia-causing SEL1L variant in humans
Ataxia Type: Novel hereditary human ataxia
SEL1L-HRD1 mediated Endoplasmic Reticulum (ER) Associated Degradation (ERAD) is a principal ER protein quality control system. We identified a SEL1L mutation that disrupted ERAD function and caused severe early onset cerebellar ataxia in human patients. Determining the significance of this SEL1L mutation can shed light on the pathogenesis of cerebellar ataxia and provide novel therapeutic strategies for these patients.

Changwoo Lee
Yale University
Identifying the Role of Oligodendroglia in Spinocerebellar Ataxia Type 1 (SCA1) Pathology and Progression
Ataxia Type: SCA1
Spinocerebellar ataxia type 1 (SCA1) is a hereditary neurodegenerative disease characterized by progressive muscle weakening and gait impairment, yet no active therapeutics exist to effectively slow disease onset or progression. While various cell types are affected in this disease, the contribution of cell types beyond the cerebellar Purkinje cells is not well understood. Interestingly, we recently identified dysfunctions in oligodendrocyte progenitor cells (OPCs) and oligodendrocytes (OLs) in SCA1 that precede Purkinje cell degeneration. In this study, we will aim to elucidate the contribution of oligodendroglia to SCA1 and will determine the mechanisms underlying oligodendroglial dysfunctions, revealing novel potential entry points for therapeutic intervention in various types of ataxias and other neurodegenerative disorders.

Alyssa Soles
University of Minnesota
Characterizing Respiratory Dysfunction in Mouse Models of Spinocerebellar Ataxia Type 1
Ataxia Type: SCA1
Spinocerebellar ataxia type 1 (SCA1) is characterized by degeneration of the brain and spinal cord and in this disorder patients most frequently succumb to problems with swallowing and breathing. This project aims to investigate the underlying cause of breathing dysfunction using mouse models of Spinocerebellar ataxia type 1 (SCA1). The mutant gene that causes SCA1 is widely expressed throughout the body including in skeletal muscle and little is understood about how muscles might be affected by SCA1. Another aim of this project is to assess pathology in muscles from SCA1 mouse models and how this could contribute to problems with breathing. This research will have important implications for the regions that therapies will need to target to extend survival in SCA1.
View Past Years Funded Research
Thank You Donors!
The generosity of our donors allows NAF to focus on our mission to accelerate the development of treatments and a cure while working to improve the lives of those living with Ataxia. We could not offer this service to the Ataxia research community without you!










