Written by Dr. Gülin Öz
Edited by Dr. Celeste Suart
Advanced Magnetic Resonance Imaging (MRI) detects brain changes before ataxia symptoms.
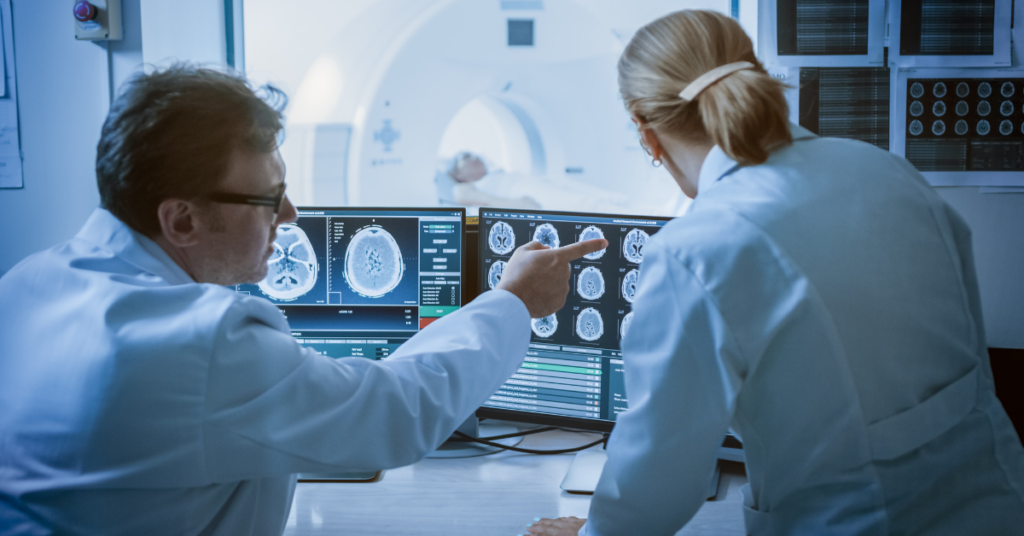
The list of pharmaceutical companies that turned their attention to ataxias has been steadily increasing over the last decade. This is in large part thanks to exciting developments in gene silencing therapies targeted to hereditary ataxias caused by toxic mutant proteins or RNA, such as the polyglutamine spinocerebellar ataxias. Because such therapies directly target what causes these diseases, they have the potential to be “disease-modifying”. This means they could, potentially, prevent the loss of neurons. Applied early enough, even before the onset of ataxia symptoms, they have the promise of preventing or delaying symptom onset altogether. Current clinical assessments, however, are not sensitive to changes at these earliest stages of ataxia. And that is where imaging comes in – to provide a window into the brain without craniotomy (drilling a hole in one’s skull)!
Magnetic resonance imaging (MRI) allows us to measure tissue loss in the brain. Loss of brain tissue is the end point of a cascade of cellular events that happen when you have ataxia. What’s more, advanced MRI methods give researchers information about events that precede the tissue loss, such as changes in the microscopic-level structure (‘microstructure’, the integrity of the wiring in the brain), chemistry, and function of the brain. The potential of non-invasive imaging measures to reliably inform us of early changes in the brain was shown by various groups starting in the late 90s. However, any biomarker that will be used in clinical trials needs to be evaluated in the trial setting. In clinical trials, the same data collection protocols are used at many locations to collect and combine harmonized data from a large group of patients.
The US-European READISCA study (Clinical Trial Readiness for SCA1 and SCA3) was designed precisely with this goal, to validate outcome measures and get ready for trials in the SCA therapeutic pipeline.
READISCA was launched in 2018 with funding from the US National Institutes of Health. Imaging is an important focus in the READISCA study. It is a longitudinal study, where participants with early SCA1 or SCA3 or those who are at risk for SCA1 or SCA3 based on their family history undergo imaging visits annually. Individuals who are at risk are tested for SCA1 or SCA3 and have the option of being told or not told about their gene status. Those at-risk participants who do not carry the SCA mutation serve as healthy controls matched to the affected individuals. Data are analyzed centrally and blinded to diagnosis, just like in a therapeutic trial. Data blinding is when the researchers analyzing the data are not told if the image they are looking at is from a healthy person or someone with ataxia. It is one way researchers minimize bias in data analysis. Quantitative outcomes are extracted from the imaging data, as standard radiological reads are not usable as biomarkers.
Chandrasekaran and colleagues recently published the first analyses of the READISCA imaging data collected at baseline, i.e. at the first visit of participants, which serves as a reference for later visits. Consistent with earlier studies, they showed that changes in peoples’ brains are detectable by MRI even at the stage before ataxia symptoms. Among the many MRI measures, the concentration of a neurochemical (total creatine in the pons) distinguished carriers of the SCA1 mutation from matched healthy controls best. A microstructure measure (diffusivity of a major neural tract that connects the cerebellum to the medulla) identified carriers of the SCA3 mutation from matched healthy controls best. It stands to reason that chemical and microstructural changes would occur earlier than structural changes in the neurodegenerative sequence of events.
In addition, the study showed that magnetic resonance metrics are strongly associated with several clinical outcome measures, including patient-reported outcomes. Hence, these non-invasive MRI measures reflect clinically meaningful outcomes for patients.
The READISCA study is an important milestone for ataxia research. READISCA demonstrated the feasibility of collecting multiple types of complex MRI data with a common research approach across multiple study locations. This is an essential step towards using these imaging techniques in upcoming clinical trials.
To summarize, this research identified the most sensitive MR markers to early brain pathology in SCA1 and SCA3. This opens the possibility of detecting disease effects in the brains of individuals before ataxia onset. Such an ability may help enroll patients in clinical trials even before ataxia signs and symptoms begin. These objective imaging measures will also be important in testing the efficacy of treatments in upcoming clinical trials. The research published in this paper is only the first step, as forthcoming long-term analyses from READISCA will be even more important for achieving these goals.
Key Words
Disease-modifying treatment: A treatment that delays or slows the progression of a disease by targeting its underlying cause.
Neurochemical: A chemical substance that is found in the nervous system and participates in neural activity.
Microstructure measure: Quantitative measurement related to the very small scale structure of the brain.
Conflict of Interest Statement
Dr. Öz serves as one of the principal investigators and the imaging lead of READISCA. Dr. Suart declares no conflict of interest.
Citation of Article Reviewed
Chandrasekaran J, Petit E, Park YW, du Montcel ST, Joers JM, Deelchand DK, Považan M, Banan G, Valabregue R, Ehses P, Faber J, Coupé P, Onyike CU, Barker PB, Schmahmann JD, Ratai EM, Subramony SH, Mareci TH, Bushara KO, Paulson H, Durr A, Klockgether T, Ashizawa T, Lenglet C, Öz G for the READISCA Consortium. Clinically meaningful MR endpoints sensitive to preataxic SCA1 and SCA3. Ann Neurol, 2022. https://onlinelibrary.wiley.com/doi/10.1002/ana.26573
Read Other SCAsource Summary Articles

¿Ser o no ser SCA27B? Esa es la cuestión: Misteriosos casos de ataxia resueltos gracias a los avances en tecnología genética
Escrito por Dr. Hayley McLoughlin y Dr. Sharan SrinivasanEditado por Dr. Celeste SuartTraducido por Ismael Araujo-Aliaga Una nueva tecnología de secuenciación genética reveló numerosos casos de ataxia familiar asociados a Read More…


Interrupciones en las repeticiones están asociadas con ataques epilépticos en SCA10
Escrito por Dr Hannah ShorrockEditado por Larissa NitschkeTraducido por Ismael Araujo-Aliaga Interrupciones en las repeticiones en SCA10 influyen en la estabilidad del tramo repetido y están asociadas con ataques epilépticos Read More…


Genetic variants in the NPTX1 gene cause cerebellar ataxia
Written by Dr. Hannah K Shorrock Edited by Dr. Celeste Suart Three genetic variants in the NPTX1 gene have been linked to cerebellar ataxia, providing a genetic diagnosis for seven Read More…









