Written by Kaitlyn Neuman
Edited by Dr Chandana Kondapalli
To induce cell death, or to degrade sick mitochondria – that is the question: a new role for ataxin-3 as a mediator of this decision.
Have you ever heard the saying: “I may look like I’m not doing anything, but on a molecular level, I’m quite busy”? Probably not. It’s pretty niche. I hope that after reading this summary, you’ll have a better understanding and appreciation for it.
Every task we do, whether a conscious decision to run or swim, or an involuntary process like maintaining your heart rate or digesting your lunch, requires a certain amount of energy. You can think of it as a currency. Things like running and swimming are going to cost more than playing a video game or taking a nap.
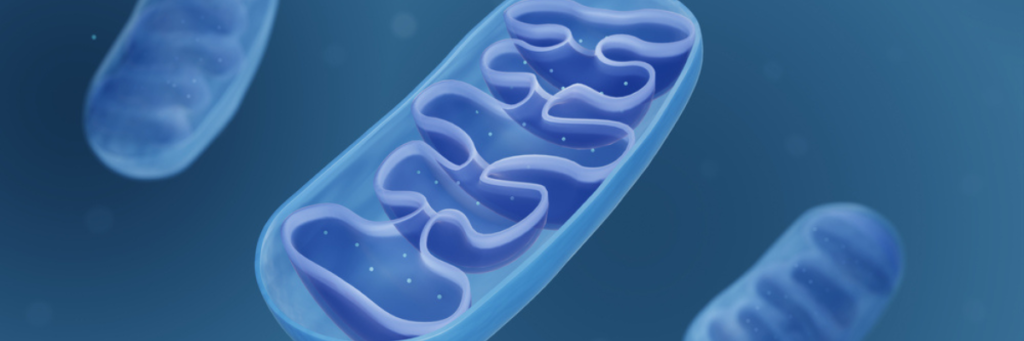
Mitochondria are the major producer of this energy currency, called ATP or adenosine triphosphate.
On the Surface Level
When your mitochondria aren’t working properly, cells starve because not enough energy is being made. Unhealthy mitochondria signal the rest of the cell to initiate death. Mitochondrial dysfunction is the cause of several common symptoms of many neurodegenerative diseases, like Spinocerebellar Ataxias (SCA). Only recently has SCA type 3 (SCA3) been linked to metabolic defects and impaired mitophagy.
Mitophagy is the process of removing damaged mitochondria to adapt to stress conditions. Removing these damaged mitochondria, before they get so sick that they need to initiate cell death, is protective for our brains and bodies. Unfortunately, sometimes in dysfunctional situations, mitophagy is blocked or stopped, leading to uncontrolled cell death. Our brain and muscles, where there are lots of mitochondria, can become vulnerable and lead to neurodegeneration and muscular weakness under these circumstances.
Since the number and location of damaged mitochondria differ from person to person, mitochondrial symptoms in SCA3 and other neurodegenerative diseases are variable. This variability is actually a good thing because it means there is a possible way to modify the disease progression: by understanding and altering these mitochondrial differences.
At the Molecular Level
The mutant SCA3 protein ataxin-3 regulates the activity of parkin, a critical protein in mitochondrial quality control that is also connected to Parkinson’s Disease. During this process, proteins are marked with a kiss of death in a process called ubiquitination. In the context of SCA3, parkin attaches a little tag called ubiquitin to a defective mitochondrial membrane protein, like VDAC1 (more on this later). The tagging process kickstarts mitophagy by recruiting molecules that send the tagged mitochondrion for recycling. This activity is known as parkin-mediated mitophagy.
Sometimes though, parkin can’t add a ubiquitin tag to these defective proteins. Instead, parkin can actually mark itself for degradation in a process called self-ubiquitination. When this happens, the proteins needed to induce mitophagy aren’t recruited, and as a consequence, cell death is initiated.
Previous studies have shown that the ataxin-3 protein increases the rate that parkin marks itself for degradation, meaning that mitophagy is inhibited in SCA3. This provides a possible explanation for the overlapping symptoms between SCA3 and Parkinson’s Disease, because both result in cell death in areas with lots of mitochondria like the brain and muscles.
What Did the Researchers Find Out and How?
Remember the VDAC1 (voltage-dependent anion channel 1) protein I briefly mentioned earlier that is tagged by parkin for degradation? Well, it plays an important role as a gatekeeper, determining what ions can move in and out of mitochondria.
When mitochondria are healthy, they are in a polarized state, meaning that the contents of the inside are negatively charged when compared to the positively charged environment. The difference in charge is what lets VDAC1 be an effective gatekeeper, moving things back and forth. On the other hand, when mitochondria are sick or damaged, they become depolarized, where the inside becomes positive.
Scientists induced this depolarization in healthy cells to find out what happens in an average person. They found that damaged mitochondria recruit parkin to tag VDAC1 and the attached mitochondrion to induce parkin-mediated mitophagy.
To accurately assess what is happening in a SCA3 patient, skin samples were taken from people with the mutated SCA3 gene. Researchers confirmed their findings from the patient-derived SCA3 cells with well-known mouse models that contain the human mutated ataxin-3 gene. Results between these two cell lines were similar, so I’m going to generalize both the human and mouse SCA3 models to “SCA3 cells”.
In SCA3 cells, researchers found that parkin was not recruited to depolarized mitochondria, unlike in healthy models. Instead, by looking at protein concentrations, they found that mutant ataxin-3 was removing these ubiquitin tags from the VDAC1 protein in a process called deubiquitination. Deubiquitination means that these sick or damaged mitochondria aren’t being broken down, and VDAC1 starts letting in larger solutes like water and salt. The increased water in the mitochondrion causes it to swell or bubble up, ultimately leading to cell death and neurodegeneration.
To confirm this water retention theory, the researchers of this paper looked at the shape and size of the mitochondria. They found that SCA3 patients have more fragmented and circular mitochondria than their healthy counterparts. Researchers also found reduced energy production and cell health in SCA3 patients.
These confirmatory methods show that SCA3 mitochondria were sick and dying, mitophagy wasn’t responding to the stress conditions, and cell death was being initiated. These findings point to a process where SCA3 cells find a way to get around using parkin to dispose of damaged mitochondria.
Final Thoughts
Overall, ataxin-3 is an important regulator of mitochondrial function because of its role in parkin-VDAC1-mediated mitophagy. This research article directly linked the mutant ataxin SCA3 as a cause of impaired mitophagy and can help explain the mitochondrial dysfunction observed in the disease.
Mitochondrial dysregulation is the root of several common symptoms in many neurodegenerative diseases. For example, similar deficits have already been linked to SCAs type 1, type 2, and type 7 and are translated by SCAsource. Some of the earliest indicators of these diseases are weight loss or trouble maintaining weight, muscle weakness, and lethargy because many critical biological functions are being sold short for their energy demand.
The bottom line is that more research is needed to understand how these parkin-independent mitophagy pathways affect SCA3 disease pathology, and down the line could potentially lead to discoveries of high therapeutic value.
Keywords
Mitochondria: The major producer of energy in cells.
Mitophagy: The process where damaged mitochondria are recycled to adapt to stress conditions and prevent cell death.
Ubiquitination: The process where another protein is marked for degradation with a ubiquitin tag.
Self-Ubiquitination: The process where a protein can mark itself for degradation by adding ubiquitin tags to its surface.
Deubiquitination: The process where ubiquitin tags are removed from another protein before it can be degraded.
Depolarization: When the contents of the mitochondria turn from negatively charged to positively charged.
Conflict of Interest Statement
The author and editor declare no conflict of interest.
Citation of Article Reviewed
Harmuth, T., et al. Mitochondrial Dysfunction in Spinocerebellar Ataxia Type 3 Is Linked to VDAC1 Deubiquitination. International Journal of Molecular Sciences, 2022. 23(5933): p. 1-20. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9180688/pdf/ijms-23-05933.pdf)

Los científicos desarrollan un nuevo enfoque para evaluar la Ataxia en casa
Escrito por Ziyang Zhao Editador por la Dra. Hayley McLoughlin Traducido por Ismael Araujo Aliaga Una aplicación para teléfonos inteligentes recientemente desarrollada permitirá a los pacientes evaluar la ataxia en casa. Existe Read More…
The SCA2 Chronicles: Unmasking COVID-19’s impact on Mind and Movement in a Galaxy Not So Far Away
Written by Kaitlyn Neuman Edited by Celeste Suart, PhD Lessons from a global pandemic: COVID-19 negatively impacts speech function and mental health in SCA2 patients. A short time ago, in a Read More…

Online Speech Therapy program helps improve speech in ataxia
Written by Caroline Spencer, PhD Edited by Celeste Suart, PhD ClearSpeechTogether is a virtual group-based speech therapy program for people with speech problems due to progressive ataxia. In this article, researchers Read More…










