Bill Nye is back in his lab with NAF and Biogen—breaking down the science of Friedreich Ataxia in a new video series. WATCH NOW
Bill Nye is back in his lab with NAF and Biogen—breaking down the science of Friedreich Ataxia in a new video series. WATCH NOW
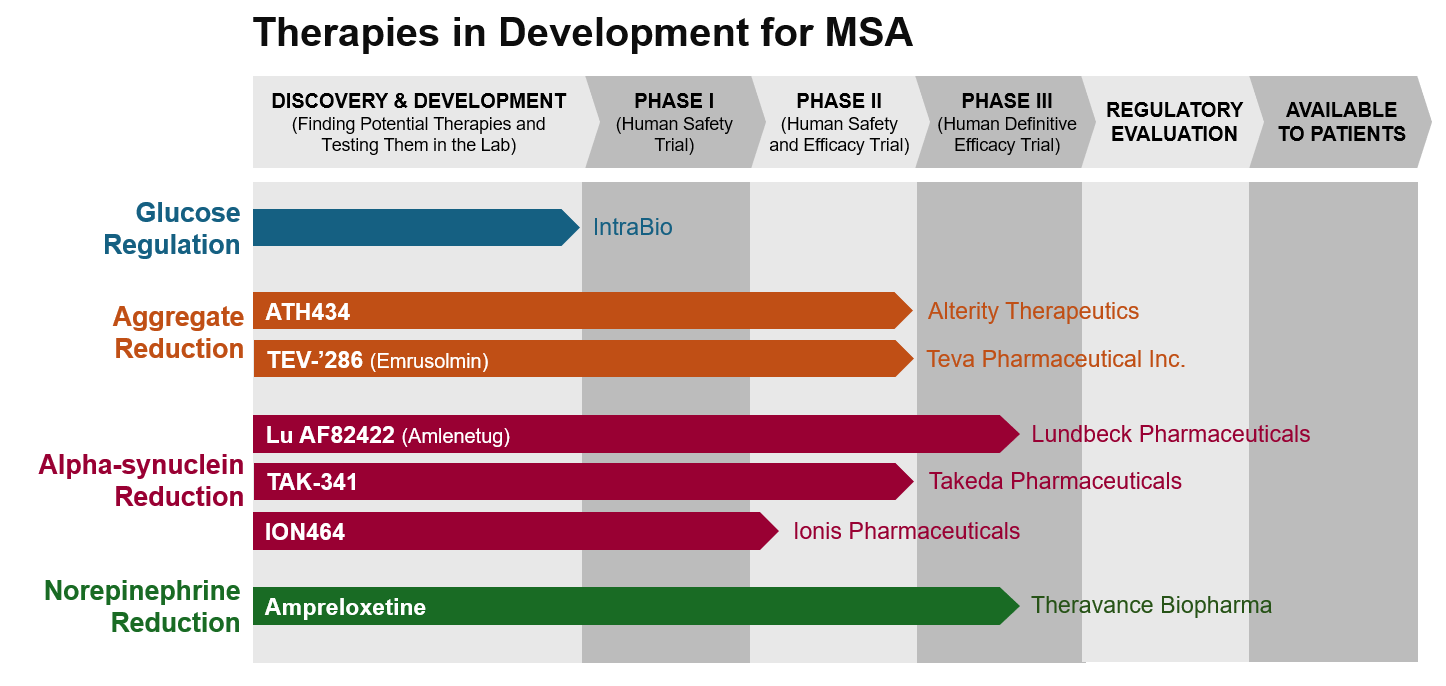
This is a visual tool created to show the progress of MSA therapies that are currently being developed. Along the right side of the diagram, treatments are grouped by their mechanism, or how the drug works. The top of the diagram shows the stage of research where the drug is in development. Phases 1 through 3 involve clinical trials with human participants. Visit our PrepRARE: Clinical Trial Readiness Education page to learn more about each phase.
Once clinical trials provide enough evidence to prove that the drug is safe and effective, the sponsor of the drug can apply for regulatory approval of the drug. After regulatory approval, the drug can become available to individuals through their healthcare provider.
The agency responsible for reviewing and approving new treatments varies depending on where you are in the world. Some regulatory agencies include:
You can click the links above to learn more about each regulatory agency. This includes information about how they approve new medications.
Please note that a drug’s status is subject to change. Please visit clinicaltrials.gov for the most current information available for specific therapies. Detailed information is available for some of the drugs listed.
IntraBio is a biopharmaceutical company developing therapies for neurodegenerative diseases with high unmet medical needs. They have Orphan Drug Designations (FDA) and Orphan Medicinal Drug Designations (EMA) for several rare diseases, including MSA.
ATH434 (Alterity Therapeutics) is an investigational oral medication that prevents α-synuclein from aggregating in the brain. By reducing the amount of aggregated α-synuclein, ATH434 restores a normal iron balance in the brain and aims to reduce MSA symptoms.
TEV-’286 (Emrusolmin, Teva Pharmaceutical Inc.) is an investigational oral medication that prevents α-synuclein from aggregating in the brain. By reducing the amount of aggregated α-synuclein, TEV-’286 restores a normal iron balance in the brain and aims to reduce MSA symptoms.
Lu AF82422 (Amlenetug) is an investigational monoclonal antibody treatment for MSA. Lu AF82422 is designed to bind to α-synuclein, which aims to reduce MSA symptoms by decreasing the amount of α-synuclein in cells.
TAK-341 (Takeda Pharmaceuticals) is an investigational monoclonal antibody treatment for MSA. TAK-341 is designed to bind to α-synuclein, which aims to reduce MSA symptoms by decreasing the amount of α-synuclein in cells.
ION464 (Ionis Pharmaceuticals) is an investigational antisense oligonucleotide (ASO) therapy. It prevents the production of α-synuclein protein. This reduces the amount of α-synuclein in cells, which aims to reduce MSA symptoms.
Ampreloxetine (Theravance Biopharma) is an investigational oral medication that treats symptomatic neurogenic orthostatic hypotension (nOH), a condition where a drop in blood pressure occurs when someone stands up. nOH can cause lightheadedness, dizziness, or fainting. Ampreloxetine is designed to block the reabsorption of norepinephrine in the brain, which helps maintain a person’s blood pressure. nOH is often experienced by people with MSA, and Ampreloxetine is being tested to treat this particular symptom.
To learn more about participating in research visit our PrepRARE Clinical Trial Readiness Education page.
