Bill Nye the Science Guy is speaking at the 2024 Annual Ataxia Conference! Register now. LEARN MORE!
Bill Nye the Science Guy is speaking at the 2024 Annual Ataxia Conference! Register now. LEARN MORE!
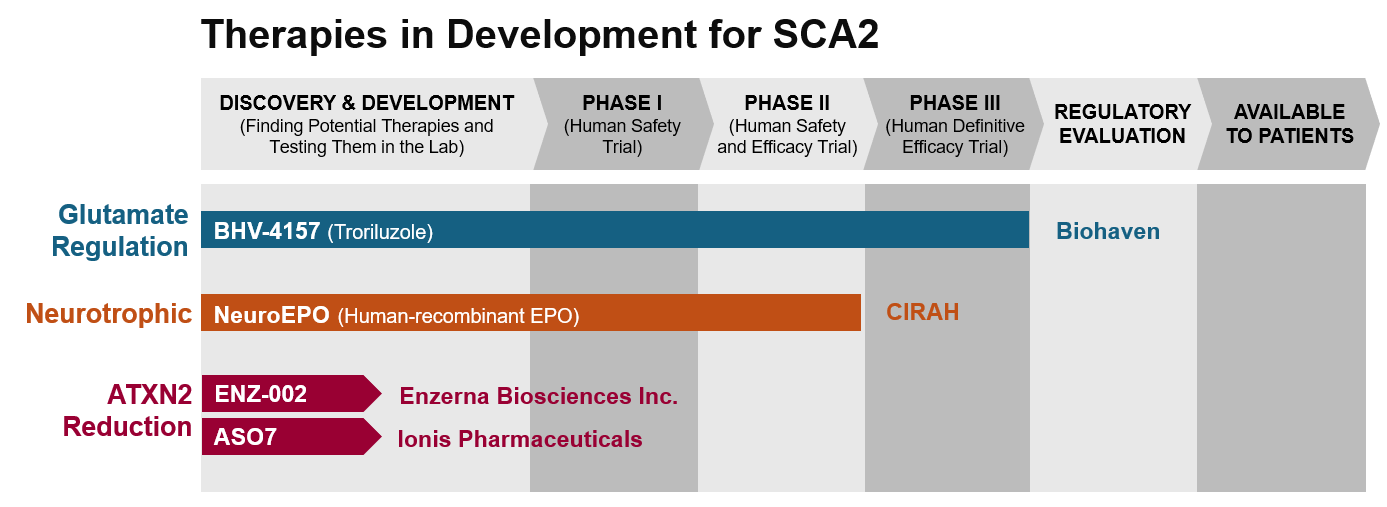
This is a visual tool created to show the progress of SCA2 therapies that are currently being developed. Along the right side of the diagram, treatments are grouped by their mechanism, or how the drug works. The top of the diagram shows the stage of research where the drug is in development. Phases 1 through 3 involve clinical trials with human participants. Visit our PrepRARE: Clinical Trial Readiness Education page to learn more about each phase.
Once clinical trials provide enough evidence to prove that the drug is safe and effective, the sponsor of the drug can apply for regulatory approval of the drug. After regulatory approval, the drug can become available to individuals through their healthcare provider.
The agency responsible for reviewing and approving new treatments varies depending on where you are in the world. Some regulatory agencies include:
You can click the links above to learn more about each regulatory agency. This includes information about how they approve new medications.
Please note that a drug’s status is subject to change. Please visit clinicaltrials.gov for the most current information available for specific therapies. Detailed information is available for some of the drugs listed.
To learn more about participating in research visit our PrepRARE Clinical Trial Readiness Education page.
Our generous donors help us fund promising Ataxia research and offer support services to people with Ataxia. Your gift today will help us continue to deliver on our mission to improve the lives of persons affected by Ataxia.
Join for FREE today! Become a part of the community that is working together to find a cure. As a member you will receive access to the latest Ataxia news with our e-newsletter and Generations publication.
