Written by Dr Hannah K Shorrock
Edited by Dr Larissa Nitschke
Splice modifying compounds can regulate whether or not gene therapy is active
Gene therapy is an incredibly versatile therapeutic strategy that can be used to treat monogenic disorders. Gene therapy can inactivate or silence mutant gene transcripts, edit the genome to repair mutated genes, or express healthy copies of disease genes. Gene therapy is often delivered using a viral vector, but this can lead to various issues such as a lack of control over the levels of expression, alterations to the DNA or RNA other than the desired effects, and the viral vectors becoming part of the human DNA. These adverse effects could be minimized if the gene therapy expression could be tightly controlled. This is what the team led by Dr. Davidson set out to do: they developed a switch to control whether gene therapy is active or if it is switched off.
The group started their pursuit of a gene therapy switch by identifying small molecules capable of altering splicing. Splicing is the process in which a newly made messenger RNA transcript is converted into a mature messenger RNA. During splicing, sections of genetic code in the newly made messenger RNA called introns are removed from between sections called exons. By splicing the exons back together a mature mRNA is made. There are small molecules approved for treating the childhood-onset motor neuron disease, spinal muscular atrophy, that act by binding across a position where a newly synthesized mRNA transcript would normally be spliced to generate the mature transcript. The group took advantage of this system to control the inclusion or exclusion of genetic material that signals whether or not a protein should be made from the RNA transcript. If no protein is produced, then the gene therapy would not be expressed and would be in an off state, if a protein is made, then the gene therapy would be active or switched on.
The group first needed to find a genetic ‘context’ that was responsive to the splice-switching small molecule when used at a low dose. After treating cells with the compound called LMI070, the group looked for changes in splicing patterns compared to untreated cells. The group found 45 events changed in the splicing of control versus LMI070 treated cells. These events included a splicing change in the gene SF3B3. By re-analyzing published RNA sequencing data, they found that many of these events, including SF3BS, were consistently altered. Next, the group looked at how frequently these splicing changes occurred without LIM070 treatment. By analyzing these 45 events in 21,504 human RNA-seq datasets, the team found that the LMI070-induced change in SF3B3 rarely occurs naturally (without the presence of LMI070). Finally, the group found that treatment with LMI070, at the dose that leads to robust SF3B3 splicing changes, caused minimal changes in gene expression. Only five upregulated and nine downregulated genes were identified. This work showed that the SF3B3 splicing event is a highly selective genetic ‘context’ responsive to low doses of LMI070 and that LMI070 treatment caused minimal off-target effects – a highly sought-after characteristic for gene therapy switches.
To achieve control of gene therapy, the group made a synthetic exon that contains the signal for where to start making a protein. They placed this synthetic exon within the genetic context of SF3B3 which responds to LMI070. This combined genetic sequence was called Xon. In this system, LMI070 treatment led to the inclusion of the synthetic exon in the mature mRNA and the production of a reporter, green fluorescent protein (GFP). The GFP reporter enables the researchers to track the level of control they have over the system. Without LMI070, there is no production of GFP.
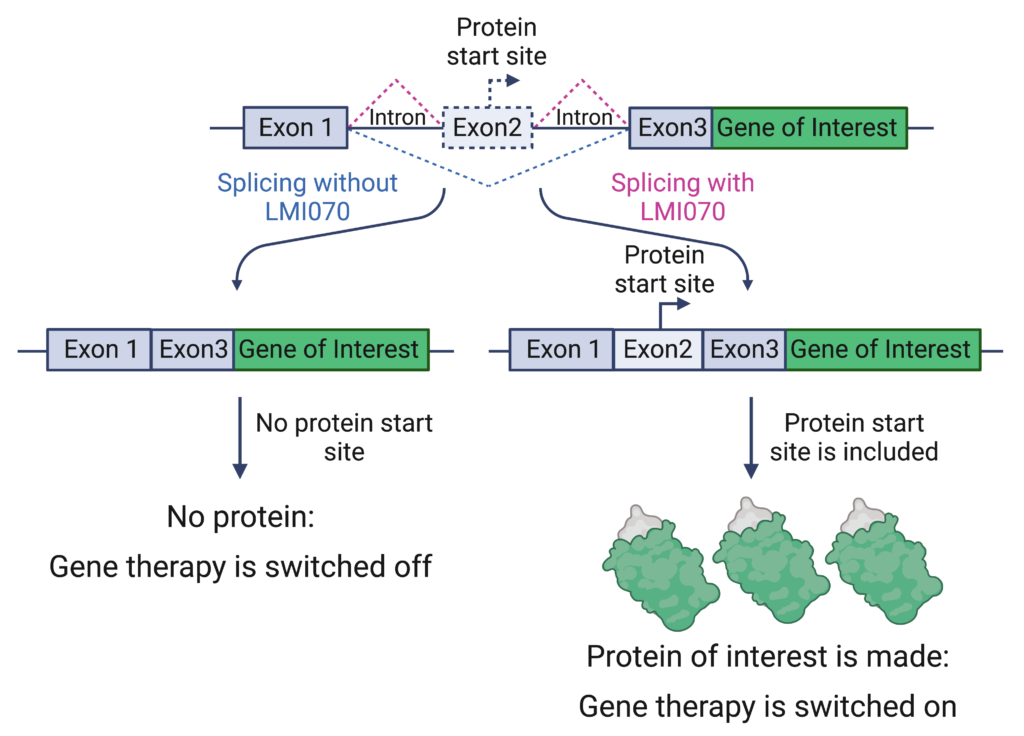
Xon system: A small molecule inducible gene therapy switch Exons 1, 3 and both introns are derived from the SF3B3 sequence that undergoes splicing changes in response to LMI070. Exon 2 is a synthetic exon that includes the signal for where to start making the protein and is only included when LMI070 is present. The gene of interest is the sequence used for gene therapy, such as erythropoietin, or a reporter, such as GFP. Dotted lines indicate which sections are spliced out with and without LMI070 treatment. The illustration was created by Dr. Hannah Shorrock and Dr. Larissa Nitschke using Biorender.com.
Using a viral AAV vector, the Xon GFP sequence was given to wild-type mice using an administration route typical for gene therapy. Four weeks later, mice were treated once with oral LMI070 or a control solution. The second group of mice received the first dose at the same time point, and one week later, they received a second oral dose of LMI070 or control solution. GFP was only detected in the mice that had received LMI070 and was detected across multiple organs, including liver, muscle, and heart in one study and brain in another. The amount of GFP detected after each dose of LMI070 was similar. Together, this indicated that the Xon -LMI070 gene therapy switch is capable of inducing robust expression of the gene therapy and that without LMI070 treatment, the gene therapy is inactive.
To test this system in a disease context, the group used an AAV Xon system to deliver erythropoietin which is used to treat anemia associated with chronic kidney disease. Following treatment with LMI070, a 25- to 62-fold increase in plasma erythropoietin levels was detected with a return to baseline levels five days after the withdrawal of LMI070. This effect was dependent on the dose of LMI070 administered. Finally, the group also tested the ability of the Xon -LMI070 switch to control the expression of CRISPR-mediated gene editing. Several weeks after intravenous administration of the Xon -CRISPR sequence, LMI070 was administered. Seven days later, the group detected the specific change in the genetic code induced by CRISPR, indicating that the Xon -LMI070 switch can be used to regulate the expression of a variety of gene therapy-based treatment approaches. Together, this shows that the level of expression of gene therapy and the time frame in which gene therapy is active can be controlled by the Xon -LMI070 switch.
By demonstrating that the Xon-LMI070 switch can be used to regulate the timing and expression levels of different gene therapy approaches across different organ systems, the group has greatly expanded the possible utility of gene therapy approaches. For some proteins, there is a very small window for which protein expression is well tolerated by the cell: too little protein may lead to one disease, while too much may have different negative consequences. While this type of switch-based system will take time to translate into the clinic, the work here provides a solid foundation for fine-tuning control of gene therapy approaches.
Keywords
Splicing: the process in which a newly made messenger RNA transcript is converted into a mature messenger RNA. During splicing, sections of genetic code in the newly made messenger RNA called introns are removed from between sections called exons. By splicing the exons back together a mature mRNA is made.
Exon: regions of the genome that are present in the mature mRNA. Proteins are coded for by the genetic material located in exons. Not all exons code for proteins.
Intron: regions located between exons that do not code for proteins. Introns interrupt the sequence that makes up a gene.
RNA-sequencing: next generation sequencing technique that quantifies which RNAs are present in a biological sample and the quantity of those RNAs.
Conflict of Interest Statement
The author and editor declare no conflict of interest.
Citation of Article Reviewed
Monteys, A. M., Hundley, A. A., Ranum, P. T., Tecedor, L., Muehlmatt, A., Lim, E., Lukashev, D., Sivasankaran, R. & Davidson, B. L. Regulated control of gene therapies by drug-induced splicing. Nature 596, 291-295, doi:10.1038/s41586-021-03770-2 (2021).

Interrupciones en las repeticiones están asociadas con ataques epilépticos en SCA10
Escrito por Dr Hannah ShorrockEditado por Larissa NitschkeTraducido por Ismael Araujo-Aliaga Interrupciones en las repeticiones en SCA10 influyen en la estabilidad del tramo repetido y están asociadas con ataques epilépticos Read More…


Genetic variants in the NPTX1 gene cause cerebellar ataxia
Written by Dr. Hannah K Shorrock Edited by Dr. Celeste Suart Three genetic variants in the NPTX1 gene have been linked to cerebellar ataxia, providing a genetic diagnosis for seven Read More…


Cientistas desenvolvem uma nova abordagem para avaliar ataxia em casa
Escrito por Ziyang ZhaoEditado por Dr. Hayley McLoughlinTraduzido para o Português por Priscila Pereira Sena Um aplicativo para celulares recentemente desenvolvido permitirá aos pacientes avaliar a ataxia em casa. Há Read More…









