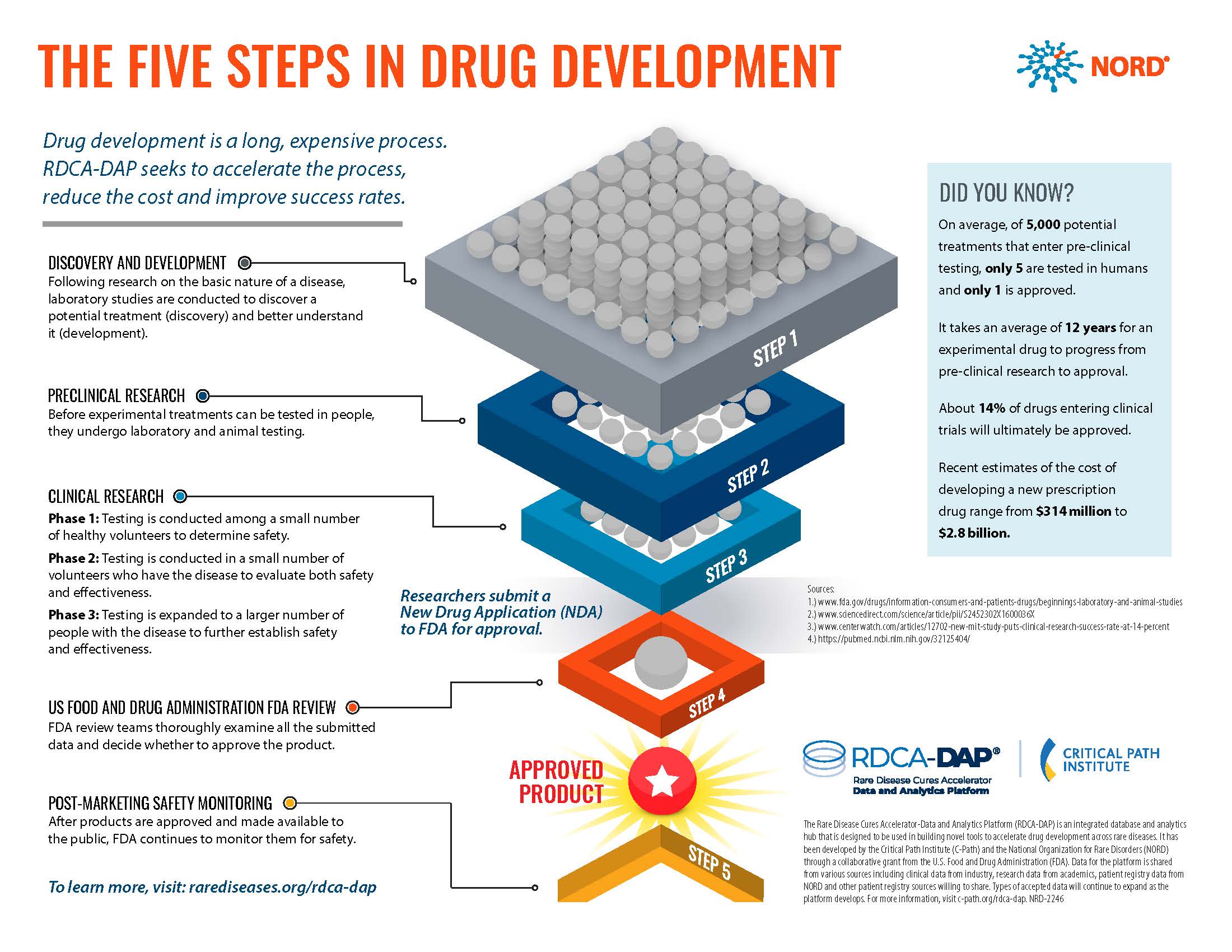
Step 1: Discovery and Development
Before a treatment can be created, researchers need to understand how a disorder impacts the body. This step is sometimes called ‘basic research’ since it focuses on asking basic questions. In the case of Ataxia, some of the questions asked include:
- What causes this type of Ataxia to happen?
- What kinds of symptoms occur?
- What does this type of Ataxia do inside people’s cells?
Once researchers have an idea of how an Ataxia type impacts the body, they can begin researching how to fix things. This discovery research takes trial and error, with thousands of potential treatments being tested. Most of this work is done using donated human tissue, cell lines, or animal models. Once they identify a potential treatment that has a measurable effect, researchers will conduct further tests to better understand how the treatment works. This step is called development.
Step 2: Preclinical Research
After several potential treatment options are identified through discovery and development, preclinical research will begin. This step is focused on safety. Researchers must check that these treatment options are safe for people to use.
Some treatments may be very effective when tested in a petri dish, but toxic when used in larger quantities. Researchers use various laboratory tools and animal models to identify unsafe or ineffective treatment options. They will also determine how long treatments stay in the human body. This is important information. It will help the researchers determine what dose of treatment to use in future tests.
On average, only 5 out of 5,000 treatments that enter pre-clinical testing move on to the next step of research.
Step 3: Clinical Research
After a treatment is shown to be safe through preclinical research, the next step is clinical research. This step involves human volunteers taking potential treatment. There are three phases of clinical research.
In Phase 1, the potential treatment is tested in a small number of healthy volunteers. This is yet another step to confirm the safety of the potential treatment. If the drug is safe, with minimal side effects, it will move to Phase 2.
In Phase 2, the potential treatment is tested in a small number of volunteers with Ataxia. This stage examines safety yet again, as well as monitors any potential side effects of the treatment.
Since Phase 2 is the first trial stage where people with Ataxia are taking the potential treatment, the researchers will also test how effective the treatment is. Different treatments have different goals, so what they measure to show effectiveness can vary.
Common measurements will include assessing balance and coordination. The researchers will monitor the increase or decrease of other Ataxia symptoms. Usually, researchers will test multiple doses of a potential treatment. This is done to find the dose that has the most benefits and fewest side effects. If a treatment is shown to be safe, have minimal side effects, and reduce Ataxia symptoms, then it will move to Phase 3.
In Phase 3, testing is expanded to a larger number of volunteers with Ataxia. The goal of Phase 3 is to confirm the results of the Phase 2 trial in a larger group of people. Researchers will again assess the safety and effectiveness of the potential treatment.
Additionally, researchers examine if the potential treatment is better than current treatment options if they exist. For many kinds of Ataxia, there are not yet other approved treatment options to compare new treatments to.
Step 4: Regulatory Review
If a potential treatment is safe and effective, it will make it through all three phases of clinical research. Then comes the next step, regulatory review. Depending on where you are in the world, different agencies are responsible for reviewing potential treatments:
- United States: The Food and Drug Administration (FDA)
- Canada: Health Canada
- Europe: The European Medicines Agency
- Australia: The Therapeutics Goods Administration
- And many more!
Although each regulatory agency has slightly different procedures for approving new treatments, they all have the same goal. They want to confirm that Steps 1-3 in the drug development process were done properly. To do this, the regulators will go through all the data provided by the developers of a potential treatment. The regulator’s job is to confirm a drug is both safe and effective.
Step 5: Post-Market Safety Monitoring
If a drug is approved by a regulatory agency, it becomes available for doctors to prescribe to patients. But the steps of drug development don’t stop there! Now that the drug is on the market, it enters Post-Market Safety Monitoring.
In this step, the regulatory agency will monitor the safety of approved treatments. They will keep track of the types and frequency of side effects associated with the treatment. If there is an unusual number of people having major side effects, the regulatory agency will investigate why this is happening. If necessary, the regulatory agency will remove the treatment from the market.
Want to Learn More About Drug Development?
You can learn more about the drug development process for rare diseases like ataxia through our PrepRARE Clinical Readiness Education series. Check out this talk from David Spotts on Drug Development from a Biotech perspective.
You may also be interested in the Rare Disease Drug Development: What Patients and Advocates Need to Know online course available through the National Organization for Rare Disorders (NORD). One of the case studies used in this course is the approval of SKYCLARYS™ (omaveloxolone) for Friedreich Ataxia and the advocacy work done by the Friedreich Ataxia Research Alliance.
Read Our Other PrepRARE Articles
Eligibility in Clinical Trials: Learn More about Inclusion and Exclusion Criteria

All About New Drug Applications (NDAs)

What is an Investigational New Drug (IND) application?

Patient Registries – What are they and why do they matter?
Finding Clinical Trial Information Using ClinicalTrials.Gov